Downstream Processing Of Proteins Pdf
The Malaysian Natural Rubber Industry In the last decade, the world NR industry has undergone very rapid and fundamental changes with the appearance of many new. Beginning with harvest of material from a bioreactor, downstream processing removes or reduces contaminants to acceptable levels through several steps that typically. CHT Ceramic Hydroxyapatite Instruction Manual Please read these instructions prior to using CHT ceramic hydroxyapatite. If you have any questions or comments. Peter J. Russell, iGenetics Copyright Pearson Education, Inc., publishing as Benjamin Cummings 13 Mechanism of Splicing There is an intranuclear proteinRNA. Downstream Processing Of Proteins Pdf Files' title='Downstream Processing Of Proteins Pdf Files' />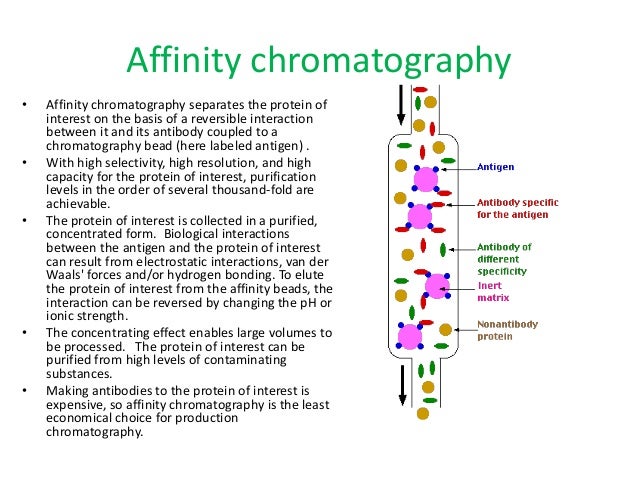 Proteasome Wikipedia1. S redirects here. It is not to be confused with S1. Cartoon representation of a proteasome. Downstream Processing Of Proteins Pdf' title='Downstream Processing Of Proteins Pdf' />Its active sites are sheltered inside the tube blue. The caps red in this case, 1. S regulatory particles on the ends regulate entry into the destruction chamber, where the protein is degraded. Top view of the proteasome above. Proteasomes are protein complexes which degrade unneeded or damaged proteins by proteolysis, a chemical reaction that breaks peptide bonds. Enzymes that help such reactions are called proteases. Proteasomes are part of a major mechanism by which cells regulate the concentration of particular proteins and degrade misfolded proteins. Biography. Jenny Schneider received her M. How To Apps On Sharp Smart Tv. Sc. degree in Material and Nanochemistry in 2011 from the Gottfried Wilhelm Leibniz University Hannover. Proteins are targeted for degradation by the proteasome with covalent modification of a lysine residue that requires the coordinated reactions of three enzymes. Antibody characteristics Polyclonal 1. Plants Vs Zombies 2 Game Setup For Pc here. Introduction The use of immobilized streptavidin on microwell plates and beads both nonmagnetic and magnetic is prolific in scientific assays. HELSINKI UNIVERSITY OF TECHNOLOGY Faculty of Chemistry and Materials Sciences Raisa Vermasvuori PRODUCTION OF RECOMBINANT PROTEINS AND MONOCLONAL ANTIBODIES. Proteins are tagged for degradation with a small protein called ubiquitin. The tagging reaction is catalyzed by enzymes called ubiquitin ligases. Once a protein is tagged with a single ubiquitin molecule, this is a signal to other ligases to attach additional ubiquitin molecules. The result is a polyubiquitin chain that is bound by the proteasome, allowing it to degrade the tagged protein. The degradation process yields peptides of about seven to eight amino acids long, which can then be further degraded into shorter amino acid sequences and used in synthesizing new proteins. Proteasomes are found inside all eukaryotes and archaea, and in some bacteria. In eukaryotes, proteasomes are located in the nucleus and the cytoplasm. In structure, the proteasome is a cylindrical complex containing a core of four stacked rings forming a central pore. Each ring is composed of seven individual proteins. The inner two rings are made of seven subunits that contain three to seven protease active sites. These sites are located on the interior surface of the rings, so that the target protein must enter the central pore before it is degraded. The outer two rings each contain seven subunits whose function is to maintain a gate through which proteins enter the barrel. These subunits are controlled by binding to cap structures or regulatory particles that recognize polyubiquitin tags attached to protein substrates and initiate the degradation process. The overall system of ubiquitination and proteasomal degradation is known as the ubiquitin proteasome system. The proteasomal degradation pathway is essential for many cellular processes, including the cell cycle, the regulation of gene expression, and responses to oxidative stress. S0734975015000750-gr2.jpg' alt='Downstream Processing Of Proteins Pdf Notes' title='Downstream Processing Of Proteins Pdf Notes' />The importance of proteolytic degradation inside cells and the role of ubiquitin in proteolytic pathways was acknowledged in the award of the 2. Nobel Prize in Chemistry to Aaron Ciechanover, Avram Hershko and Irwin Rose. University Of Idaho Food Science Program here. DiscoveryeditBefore the discovery of the ubiquitin proteasome system, protein degradation in cells was thought to rely mainly on lysosomes, membrane bound organelles with acidic and protease filled interiors that can degrade and then recycle exogenous proteins and aged or damaged organelles. However, work by Alfred Goldberg in 1. ATP dependent protein degradation in reticulocytes, which lack lysosomes, suggested the presence of a second intracellular degradation mechanism. This was shown in 1. Later work on modification of histones led to the identification of an unexpected covalent modification of the histone protein by a bond between a lysine side chain of the histone and the C terminalglycine residue of ubiquitin, a protein that had no known function. It was then discovered that a previously identified protein associated with proteolytic degradation, known as ATP dependent proteolysis factor 1 APF 1, was the same protein as ubiquitin. The proteolytic activities of this system was isolated as a multi protein complex originally called the multi catalytic proteinase complex by Sherwin Wilk and Marion Orlowski. Later, the ATP dependent proteolytic complex that was responsible for ubiquitin dependent protein degradation was discovered and was called the 2. S proteasome. 1. 01. Much of the early work leading up to the discovery of the ubiquitin proteasome system occurred in the late 1. Mixing technology, An introduction pdf Case Versatile engineering solution for infant formula facility pdf Case Unique spray dryer pilot product development. Technion in the laboratory of Avram Hershko, where Aaron Ciechanover worked as a graduate student. Hershkos year long sabbatical in the laboratory of Irwin Rose at the Fox Chase Cancer Center provided key conceptual insights, though Rose later downplayed his role in the discovery. The three shared the 2. Nobel Prize in Chemistry for their work in discovering this system. Although electron microscopy data revealing the stacked ring structure of the proteasome became available in the mid 1. X ray crystallography until 1. Structure and organizationedit. A schematic diagram of the proteasome 2. S core particle viewed from one side. The subunits that make up the outer two rings are shown in green, and the subunits that make up the inner two rings are shown in blue. The proteasome subcomponents are often referred to by their Svedberg sedimentation coefficient denoted S. The proteasome most exclusively used in mammals is the cytosolic 2. S proteasome, which is about 2. Da in molecular mass containing one 2. S protein subunit and two 1. S regulatory cap subunits. The core is hollow and provides an enclosed cavity in which proteins are degraded openings at the two ends of the core allow the target protein to enter. Each end of the core particle associates with a 1. S regulatory subunit that contains multiple ATPaseactive sites and ubiquitin binding sites it is this structure that recognizes polyubiquitinated proteins and transfers them to the catalytic core. An alternative form of regulatory subunit called the 1. S particle can associate with the core in essentially the same manner as the 1. S particle the 1. S may play a role in degradation of foreign peptides such as those produced after infection by a virus. S core particleeditThe number and diversity of subunits contained in the 2. S core particle depends on the organism the number of distinct and specialized subunits is larger in multicellular than unicellular organisms and larger in eukaryotes than in prokaryotes. All 2. 0S particles consist of four stacked heptameric ring structures that are themselves composed of two different types of subunits subunits are structural in nature, whereas subunits are predominantly catalytic. The outer two rings in the stack consist of seven subunits each, which serve as docking domains for the regulatory particles and the alpha subunits N termini form a gate that blocks unregulated access of substrates to the interior cavity. The inner two rings each consist of seven subunits and contain the protease active sites that perform the proteolysis reactions. Three distinct catalytic activities were identified in the purified complex chymotrypsin like, trypsin like and peptidylglutamyl peptide hydrolyzing. The size of the proteasome is relatively conserved and is about 1. The interior chamber is at most 5. In archaea such as Thermoplasma acidophilum, all the and all the subunits are identical, whereas eukaryotic proteasomes such as those in yeast contain seven distinct types of each subunit. In mammals, the 1, 2, and 5 subunits are catalytic although they share a common mechanism, they have three distinct substrate specificities considered chymotrypsin like, trypsin like, and peptidyl glutamyl peptide hydrolyzing PHGH.
Proteasome Wikipedia1. S redirects here. It is not to be confused with S1. Cartoon representation of a proteasome. Downstream Processing Of Proteins Pdf' title='Downstream Processing Of Proteins Pdf' />Its active sites are sheltered inside the tube blue. The caps red in this case, 1. S regulatory particles on the ends regulate entry into the destruction chamber, where the protein is degraded. Top view of the proteasome above. Proteasomes are protein complexes which degrade unneeded or damaged proteins by proteolysis, a chemical reaction that breaks peptide bonds. Enzymes that help such reactions are called proteases. Proteasomes are part of a major mechanism by which cells regulate the concentration of particular proteins and degrade misfolded proteins. Biography. Jenny Schneider received her M. How To Apps On Sharp Smart Tv. Sc. degree in Material and Nanochemistry in 2011 from the Gottfried Wilhelm Leibniz University Hannover. Proteins are targeted for degradation by the proteasome with covalent modification of a lysine residue that requires the coordinated reactions of three enzymes. Antibody characteristics Polyclonal 1. Plants Vs Zombies 2 Game Setup For Pc here. Introduction The use of immobilized streptavidin on microwell plates and beads both nonmagnetic and magnetic is prolific in scientific assays. HELSINKI UNIVERSITY OF TECHNOLOGY Faculty of Chemistry and Materials Sciences Raisa Vermasvuori PRODUCTION OF RECOMBINANT PROTEINS AND MONOCLONAL ANTIBODIES. Proteins are tagged for degradation with a small protein called ubiquitin. The tagging reaction is catalyzed by enzymes called ubiquitin ligases. Once a protein is tagged with a single ubiquitin molecule, this is a signal to other ligases to attach additional ubiquitin molecules. The result is a polyubiquitin chain that is bound by the proteasome, allowing it to degrade the tagged protein. The degradation process yields peptides of about seven to eight amino acids long, which can then be further degraded into shorter amino acid sequences and used in synthesizing new proteins. Proteasomes are found inside all eukaryotes and archaea, and in some bacteria. In eukaryotes, proteasomes are located in the nucleus and the cytoplasm. In structure, the proteasome is a cylindrical complex containing a core of four stacked rings forming a central pore. Each ring is composed of seven individual proteins. The inner two rings are made of seven subunits that contain three to seven protease active sites. These sites are located on the interior surface of the rings, so that the target protein must enter the central pore before it is degraded. The outer two rings each contain seven subunits whose function is to maintain a gate through which proteins enter the barrel. These subunits are controlled by binding to cap structures or regulatory particles that recognize polyubiquitin tags attached to protein substrates and initiate the degradation process. The overall system of ubiquitination and proteasomal degradation is known as the ubiquitin proteasome system. The proteasomal degradation pathway is essential for many cellular processes, including the cell cycle, the regulation of gene expression, and responses to oxidative stress. S0734975015000750-gr2.jpg' alt='Downstream Processing Of Proteins Pdf Notes' title='Downstream Processing Of Proteins Pdf Notes' />The importance of proteolytic degradation inside cells and the role of ubiquitin in proteolytic pathways was acknowledged in the award of the 2. Nobel Prize in Chemistry to Aaron Ciechanover, Avram Hershko and Irwin Rose. University Of Idaho Food Science Program here. DiscoveryeditBefore the discovery of the ubiquitin proteasome system, protein degradation in cells was thought to rely mainly on lysosomes, membrane bound organelles with acidic and protease filled interiors that can degrade and then recycle exogenous proteins and aged or damaged organelles. However, work by Alfred Goldberg in 1. ATP dependent protein degradation in reticulocytes, which lack lysosomes, suggested the presence of a second intracellular degradation mechanism. This was shown in 1. Later work on modification of histones led to the identification of an unexpected covalent modification of the histone protein by a bond between a lysine side chain of the histone and the C terminalglycine residue of ubiquitin, a protein that had no known function. It was then discovered that a previously identified protein associated with proteolytic degradation, known as ATP dependent proteolysis factor 1 APF 1, was the same protein as ubiquitin. The proteolytic activities of this system was isolated as a multi protein complex originally called the multi catalytic proteinase complex by Sherwin Wilk and Marion Orlowski. Later, the ATP dependent proteolytic complex that was responsible for ubiquitin dependent protein degradation was discovered and was called the 2. S proteasome. 1. 01. Much of the early work leading up to the discovery of the ubiquitin proteasome system occurred in the late 1. Mixing technology, An introduction pdf Case Versatile engineering solution for infant formula facility pdf Case Unique spray dryer pilot product development. Technion in the laboratory of Avram Hershko, where Aaron Ciechanover worked as a graduate student. Hershkos year long sabbatical in the laboratory of Irwin Rose at the Fox Chase Cancer Center provided key conceptual insights, though Rose later downplayed his role in the discovery. The three shared the 2. Nobel Prize in Chemistry for their work in discovering this system. Although electron microscopy data revealing the stacked ring structure of the proteasome became available in the mid 1. X ray crystallography until 1. Structure and organizationedit. A schematic diagram of the proteasome 2. S core particle viewed from one side. The subunits that make up the outer two rings are shown in green, and the subunits that make up the inner two rings are shown in blue. The proteasome subcomponents are often referred to by their Svedberg sedimentation coefficient denoted S. The proteasome most exclusively used in mammals is the cytosolic 2. S proteasome, which is about 2. Da in molecular mass containing one 2. S protein subunit and two 1. S regulatory cap subunits. The core is hollow and provides an enclosed cavity in which proteins are degraded openings at the two ends of the core allow the target protein to enter. Each end of the core particle associates with a 1. S regulatory subunit that contains multiple ATPaseactive sites and ubiquitin binding sites it is this structure that recognizes polyubiquitinated proteins and transfers them to the catalytic core. An alternative form of regulatory subunit called the 1. S particle can associate with the core in essentially the same manner as the 1. S particle the 1. S may play a role in degradation of foreign peptides such as those produced after infection by a virus. S core particleeditThe number and diversity of subunits contained in the 2. S core particle depends on the organism the number of distinct and specialized subunits is larger in multicellular than unicellular organisms and larger in eukaryotes than in prokaryotes. All 2. 0S particles consist of four stacked heptameric ring structures that are themselves composed of two different types of subunits subunits are structural in nature, whereas subunits are predominantly catalytic. The outer two rings in the stack consist of seven subunits each, which serve as docking domains for the regulatory particles and the alpha subunits N termini form a gate that blocks unregulated access of substrates to the interior cavity. The inner two rings each consist of seven subunits and contain the protease active sites that perform the proteolysis reactions. Three distinct catalytic activities were identified in the purified complex chymotrypsin like, trypsin like and peptidylglutamyl peptide hydrolyzing. The size of the proteasome is relatively conserved and is about 1. The interior chamber is at most 5. In archaea such as Thermoplasma acidophilum, all the and all the subunits are identical, whereas eukaryotic proteasomes such as those in yeast contain seven distinct types of each subunit. In mammals, the 1, 2, and 5 subunits are catalytic although they share a common mechanism, they have three distinct substrate specificities considered chymotrypsin like, trypsin like, and peptidyl glutamyl peptide hydrolyzing PHGH.